
Laboratory Investigations in Microbiology

 |
Laboratory Investigations in Microbiology |
 |
Now that we have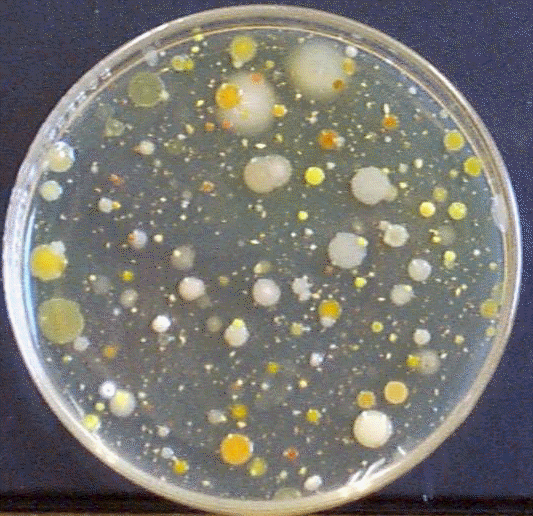 observed known microorganisms under fairly
"controlled" conditions in the laboratory, we will turn our
attention to the diversity of microbes found in our environment. Our task will
be to collect samples (soil, water, etc) from outside and to try to determine
the number of bacteria and fungi in these samples. In this process, we will
also get a look at some of the diverse organisms we can find in these
environments.
observed known microorganisms under fairly
"controlled" conditions in the laboratory, we will turn our
attention to the diversity of microbes found in our environment. Our task will
be to collect samples (soil, water, etc) from outside and to try to determine
the number of bacteria and fungi in these samples. In this process, we will
also get a look at some of the diverse organisms we can find in these
environments.
Soil is home to literally billions of microorganisms per gram. It is one of the richest microbial habitats (aside from, perhaps, the animal digestive tract), including aerobes and anaerobes, endosporing bacteria, molds, nitrogen fixers, denitrifiers, lithotrophs, decomposers, gliding bacteria, and many more. In soil these microbes participate in essential nutrient cycles, such as those for nitrogen, sulfur, and carbon. Autotophic bacteria (phototrophs and lithotrophs) fix carbon dioxide; decomposers return it to the environment. Of particular importance are bacteria that can break down complex plant carbohydrates such as starch (Bacillus) and cellulose (Cytophaga). Nitrogen fixers (Azotobacter, Rhizobium) add nitrogen to the soil; lithotrophs oxidize it to nitrate (Nitrobacter, Ntrosomonas), and denitrifiers (Pseudomonas) may remove it again. Other very abundant soil bacteria include the actinomycetes, bacteria that form fungus-like filaments and spores. Typical soils may contain from 1 million to 10 billion microbes per gram. To determine these numbers, a soil sample will be diluted up to 1 billion-fold and cultured using the pour-plate method. The resulting colonies will be counted (= viable plate count method).
However, the culturing of environmental microbes in the lab is not always successful. many microbes cannot be cultured in the laboratory even though they are alive when collected. Such microbes are called "VBNC" (viable but not culturable), and make up as much as 99% of all microbes in the environment! Some of the reasons that they cannot be cultured include special nutrient requirements (trace elements, vitamins/growth factors), unique growth conditions (pH, temperature, oxygen), the need for a cooperative community structure of other organisms or the requirement for a precise combination of all these factors, such as nutrient gradients found in soil.
Aquatic samples are home to many different microbes, including algae, cyanobacteria, enterobacteria, soil bacteria, and several species found only in water. Microbes such as Caulobacter, Beggiatoa, and Hyphomicrobium often make water their home. There are comparatively fewer culturable microbes in water than in soil - up to several thousand per 100 ml - and there are far fewer fungi. However, because of its importance as a source of drinking water, aquatic samples are usually monitored closely for fecal contamination. Although usually the Most Probable Number (MPN) test is used to estimate microbial (heterotrophic) numbers in water, today we will use the pour-plate (viable plate count) method to get a more accurate number.
Collect a sample of soil from outside with your sterile screw cap tube. Use the tube to dig into the soil. Suggestions are: flower beds, lawn, wooded lots, trees...
Next lab
© 2003 - 2019 Josť de Ondarza, Ph.D.