
Laboratory Investigations in Microbiology

 |
Laboratory Investigations in Microbiology |
 |
 Perhaps one of the most serious environmental disasters occurred long ago
on earth when a deadly poison gas was (deliberately?) released into the
earth's atmosphere, killing many of its inhabitants. The poison gas? Oxygen!
To you and me, oxygen is, of course, vital for respiration. At one point in
time, though, the earth's atmosphere was likely completely anaerobic,
containing such reducing compounds as hydrogen sulfide gas, ammonium, and
hydrogen gas. Bacteria that lived then were presumably strict anaerobes. Then
along came cyanobacteria - oxygen-producing photoautotrophs that gradually
turned the atmosphere into what we are familiar with today. Although
eventually algae and plants contributed to this process, one may say that it
is ultimately still cyanobacteria (chloroplasts by endosymbiosis) that are at
work.
Perhaps one of the most serious environmental disasters occurred long ago
on earth when a deadly poison gas was (deliberately?) released into the
earth's atmosphere, killing many of its inhabitants. The poison gas? Oxygen!
To you and me, oxygen is, of course, vital for respiration. At one point in
time, though, the earth's atmosphere was likely completely anaerobic,
containing such reducing compounds as hydrogen sulfide gas, ammonium, and
hydrogen gas. Bacteria that lived then were presumably strict anaerobes. Then
along came cyanobacteria - oxygen-producing photoautotrophs that gradually
turned the atmosphere into what we are familiar with today. Although
eventually algae and plants contributed to this process, one may say that it
is ultimately still cyanobacteria (chloroplasts by endosymbiosis) that are at
work.
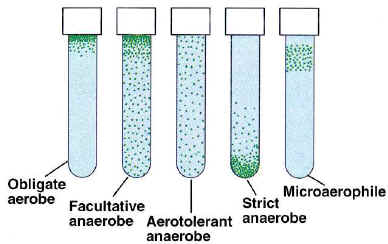
Over time, organisms, of course, adapt or perish. Strategies that have evolved to deal with an oxygen-containing atmosphere include aerobic respiration, facultatively anaerobic metabolism, aerotolerance, and various enzymes to protect cells from the damaging oxygen molecules, such as catalase and superoxide dismutase. Organisms that could not adapt were banished into anaerobic environments (deep in soils, under water or in intestinal tracts!). This demonstration exercise will show you the four most common patterns of growth in relation to oxygen.
Obligate aerobes, of course, are bacteria that require oxygen to live. Oxygen is used in aerobic respiration as the sole electron acceptor, and its absence dooms the aerobe to death. Obligate aerobes are, not surprisingly, strictly respiratory (no fermentation) and carry the enzymes superoxide dismutase and catalase. Aerobes also have the enzyme cytochrome oxidase, which can be detected using the oxidase test. Examples of aerobes include Pseudomonas aeruginosa and Micrococcus luteus.
Obligate anaerobes, in contrast, are killed by the presence of oxygen. They have never developed a strategy for coping with this toxic compound or its byproducts, are catalase- and superoxide dismutase-negative, and can be found in nature only where little or no oxygen exists. Obligate anaerobes may be respiratory (using anaerobic respiration) or fermentative. Examples of obligate anaerobes include Clostridium botulinum and Veillonella parvula.
 Facultative
anaerobes have both a respiratory and a fermentative metabolism.
For respiration, they often have a branched electron transport chain that
allows them to use multiple electron acceptors (oxygen, nitrate, sulfate).
Because aerobic respiration is a much more efficient process than anaerobic
respiration or fermentation, facultative anaerobes grow better with oxygen than
without. Examples of facultative anaerobes include E. coli and Staphylococcus
aureus.
Facultative
anaerobes have both a respiratory and a fermentative metabolism.
For respiration, they often have a branched electron transport chain that
allows them to use multiple electron acceptors (oxygen, nitrate, sulfate).
Because aerobic respiration is a much more efficient process than anaerobic
respiration or fermentation, facultative anaerobes grow better with oxygen than
without. Examples of facultative anaerobes include E. coli and Staphylococcus
aureus.
Aerotolerant anaerobes are unaffected by oxygen. Like strict anaerobes, they do not have the ability to use oxygen. Unlike strict anaerobes, aerotolerant anaerobes are not killed by oxygen. Aerotolerant anaerobes are catalase-negative, but many do carry superoxide dismutase. These bacteria are always oxidase-negative and carry out a strictly fermentative type of metabolism. Examples in this category include Enterococcus faecalis and Streptococcus lactis.
Microaerophiles are a relatively recent discovery. These bacteria require oxygen in very small amounts and are killed by too much or too little of it. Examples include Helicobacter pylori and Campylobacter jejuni.
In order to test bacteria for their ability to grow without oxygen, an anaerobic culture chamber is required. Oxygen is removed from the chamber when it reacts with hydrogen gas to form water. By inoculating agar plates with bacteria that are incubated inside and outside of the chamber, one can determine an organism's preference for oxygen by comparing the amount of growth of these bacteria with or without oxygen.
© 2003 - 2015 Josť de Ondarza, Ph.D.